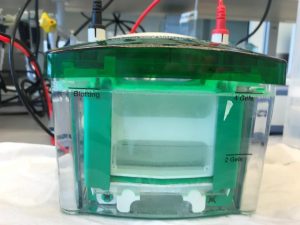
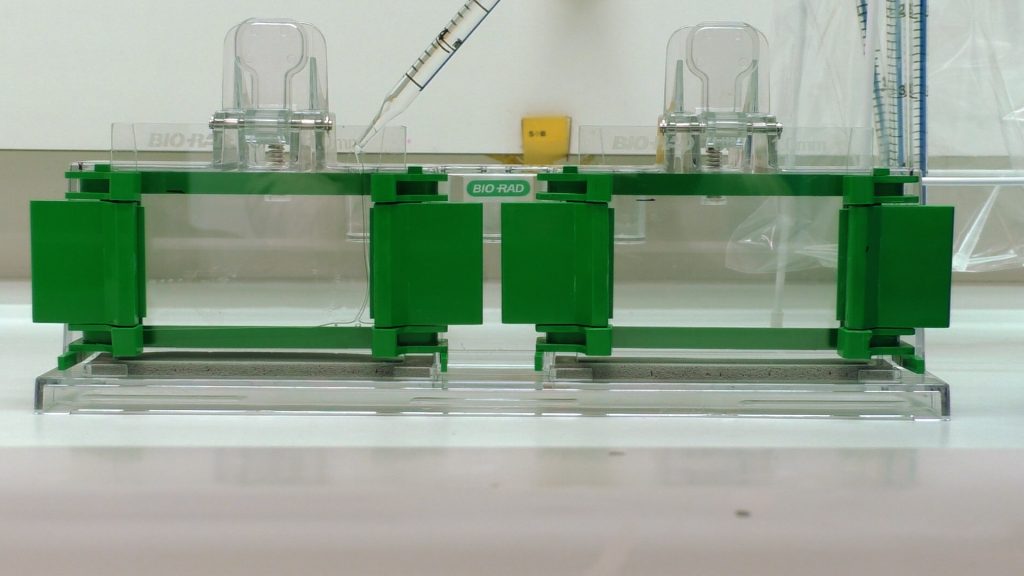
Protein separation
This video shows how to separate proteins using SDS-PAGE.
- Tineke Bijl
-
(0)
- 0 enrolled students
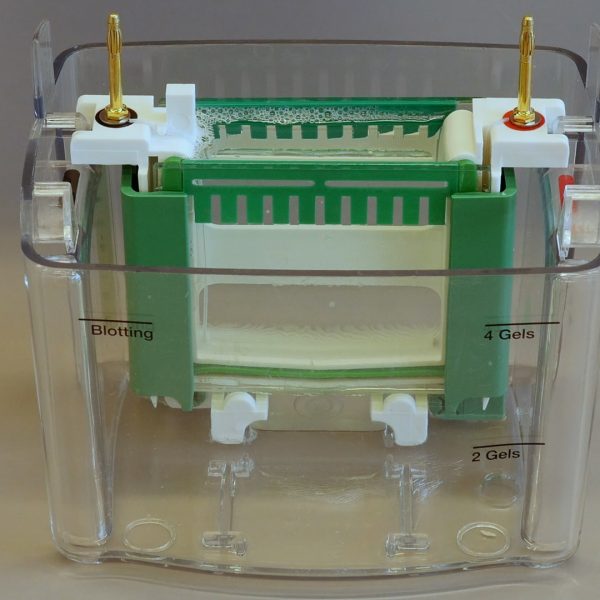
Description
Non-interactive video download link (mp4).
The script that has been used to create the video shown above can be downloaded as excel script file.
| 1 | Prepare you samples as you calculate. You need to add Sample + Ripa buffer+ SB in the right amount. |
|
| 2 |
Carefully place the gels from 1 team in the gel electrophoresis holder (note the orientation – opening of slots to the inside) and pour electrophoresis buffer:
|
|
| 3 | Carefully remove the combs from the stacking gel. | |
| 4 | Pipette 2 µl protein marker into the left slot. | |
| 5 | Pipette your samples into the wells to the right of the marker. Make a note of the order in which you pipette the samples. | |
| 6 |
Start the electrophoresis run – check for bubbles at the bottom.
|
|
| 7 |
Stop the run when the blue colored bands reach the end of the gel. First stop the program and unplug the appliance, only then remove the gels! Note: Proceed immediately with the blotting. If you have to wait for an assistant, keep your gel running on the lowest voltage (20V), so your proteins stay separated. |
|
| 8 |
Bring back used stuff back to the assistent and refill stock. Hand back to assistent:
Refill bottle with elektroforese buffer. |
|
| 9 |
Dispose waste. | |
Why is it important that there are no system leaks in the gel electrophoresis system? If in need of more explanation regarding the principle of SDS-PAGE, watch the “kennisclip” in the labbuddy environment under “gel gieten”.
|
Here you see four pictures of gels inserted into the holder. Which is correct?
|
Here you see four pictures of a gel electrophoresis at different times. Which band height correlates with the correct time to stop the electrophoresis? Knowledge of protein marker and expected band sizes is expected.
|
Wat is juist met betrekking tot de bandjes op de gel na het runnen?
|
Wat is de functie van SDS in de elektroforesebuffer?
|
Please make sure that your product exists and valid for this course
- Skill levelIntroduction video
- CategoryBiochemistry
Related videos
-
Free
Pouring SDS PAGE gel
-
Free
Blocking membrane
Copyright information
This video is created by Leiden Academic Centre for Drug Research (LACDR), Faculty of Science at Leiden University under a open Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. When using this video in its original version please refer to www.labprep.video. When adapting the video, mention the source ‘adapted based on the original version that is created by the labprep.video team’. It is not allowed to use the video for commercial purposes without consultation with the creators. You can contact us via info@labprep.video.